- 0086-571-85302990
- sales@greenskybio.com
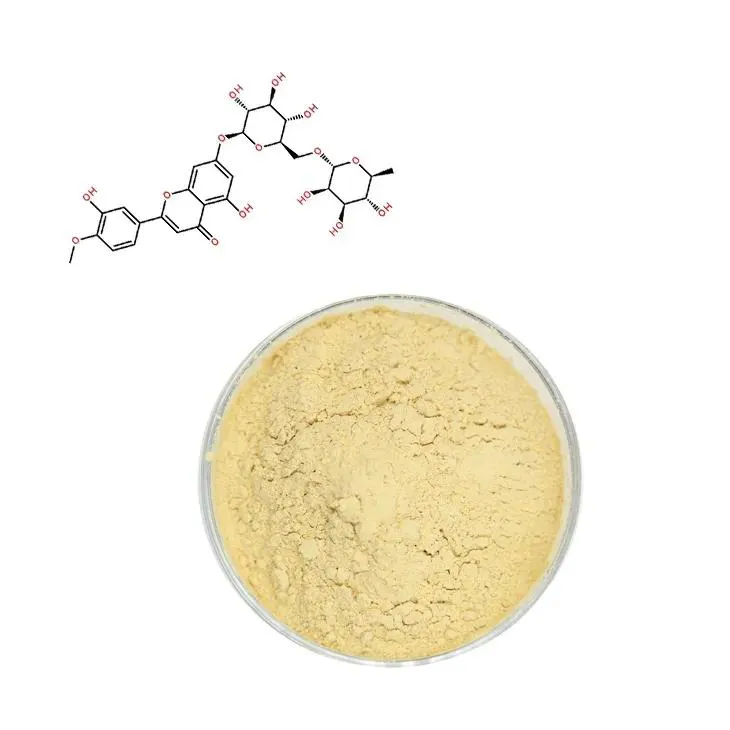
Supercritical Carbon Dioxide Extraction of Baicalin.
2024-11-30

1. Introduction
Baicalin, a flavonoid glycoside, has been recognized for its significant biological activities, such as anti - inflammatory, antioxidant, and antibacterial properties. It is mainly derived from the roots of Scutellaria baicalensis, a traditional Chinese medicinal herb. With the increasing demand for natural products in the pharmaceutical and nutraceutical industries, the extraction of Baicalin has become an important research area. Supercritical carbon dioxide (SC - CO₂) extraction has emerged as a promising technique for the extraction of Baicalin due to its several advantages over traditional extraction methods.

2. Properties of Supercritical Carbon Dioxide
2.1. Physical Properties
Supercritical carbon dioxide exists in a state above its critical temperature (31.1 °C) and critical pressure (7.38 MPa). In this state, it has unique physical properties. It has a high diffusivity, which means it can penetrate into the plant matrix quickly, facilitating the extraction process. At the same time, it has a low viscosity, which allows it to flow easily through the extraction system. These properties make supercritical carbon dioxide an excellent solvent for the extraction of bioactive compounds like baicalin.2.2. Solvent Power
The solvent power of supercritical carbon dioxide can be adjusted by changing the pressure and temperature. By manipulating these parameters, it can selectively dissolve different compounds. This selectivity is very useful in the extraction of baicalin as it can be used to separate baicalin from other components in the plant material.
3. Advantages of SC - CO₂ Extraction for Baicalin
3.1. High Extraction Yield
Due to the high diffusivity and good solvent - substrate interaction, SC - CO₂ extraction can achieve a relatively high extraction yield of baicalin. By optimizing the extraction conditions, such as pressure, temperature, and extraction time, the extraction efficiency can be further improved. For example, studies have shown that under certain optimal conditions, the extraction yield of baicalin using SC - CO₂ can be significantly higher than that of traditional solvent extraction methods.3.2. High - Quality Product
One of the major advantages of SC - CO₂ extraction is that it can produce a high - quality product. Since supercritical carbon dioxide is a clean solvent, there is no or very little solvent residue in the extracted baicalin. This is of great importance for pharmaceutical applications where the purity of the active ingredient is crucial. In addition, the mild extraction conditions used in SC - CO₂ extraction can help to preserve the integrity of baicalin and its bioactivity.3.3. Environmentally Friendly
Supercritical carbon dioxide is a non - toxic, non - flammable, and recyclable solvent. Compared to traditional organic solvents used in extraction, such as ethanol, methanol, and hexane, it has much less environmental impact. The use of SC - CO₂ extraction can reduce the consumption of organic solvents and the associated waste disposal problems, making it a more sustainable extraction method.
4. Factors Affecting SC - CO₂ Extraction of Baicalin
4.1. Pressure
Pressure is one of the most important factors affecting the SC - CO₂ extraction of baicalin. As the pressure increases, the density of supercritical carbon dioxide increases, which in turn increases its solvent power. However, too high a pressure may also lead to the extraction of unwanted components or the degradation of baicalin. Therefore, an optimal pressure needs to be determined for the extraction of baicalin. For example, research has found that in a certain range of pressure, the extraction yield of baicalin increases with increasing pressure, but beyond a certain point, the yield may start to decline or the quality of the extracted product may be affected.4.2. Temperature
Temperature also plays a crucial role in SC - CO₂ extraction. An increase in temperature can enhance the diffusivity of supercritical carbon dioxide and reduce its viscosity, which can improve the extraction efficiency. However, high temperature may cause the degradation of baicalin or other thermally sensitive components in the plant material. Thus, the temperature should be carefully controlled. For instance, some studies have indicated that there is an optimal temperature range for the extraction of baicalin using SC - CO₂, within which the extraction yield and quality are both satisfactory.4.3. Extraction Time
The extraction time is another factor that needs to be considered. Longer extraction times generally lead to higher extraction yields, but they may also increase the extraction of impurities. Moreover, extended extraction times may not be economically feasible. Therefore, an appropriate extraction time should be determined to balance the extraction yield and the purity of the product. For example, experimental results have shown that after a certain extraction time, the increase in the extraction yield of baicalin becomes marginal, and further increasing the extraction time may not be beneficial.4.4. Co - solvents
In some cases, co - solvents can be added to supercritical carbon dioxide to enhance its solvent power. For the extraction of baicalin, co - solvents such as ethanol can be used. The addition of co - solvents can change the polarity of the supercritical fluid, making it more suitable for the extraction of polar compounds like baicalin. However, the use of co - solvents also needs to be carefully optimized to avoid introducing additional impurities or affecting the properties of the final product.
5. Mechanisms of SC - CO₂ Extraction of Baicalin
The extraction of baicalin using supercritical carbon dioxide is a complex process that involves mass transfer and solubility phenomena. Firstly, supercritical carbon dioxide penetrates into the pores of the plant matrix, which is facilitated by its high diffusivity. Once inside the matrix, it solubilizes baicalin through interactions such as van der Waals forces and dipole - dipole interactions. The solubility of baicalin in supercritical carbon dioxide depends on the pressure, temperature, and the nature of the supercritical fluid. As the supercritical carbon dioxide with dissolved baicalin diffuses out of the plant matrix, the baicalin is separated from the extraction system.
6. Operational Aspects of SC - CO₂ Extraction of Baicalin
6.1. Equipment
The SC - CO₂ extraction system typically consists of a carbon dioxide source, a pump to pressurize the carbon dioxide, a temperature - controlled extraction vessel, a separator, and a collection unit. The extraction vessel is where the plant material containing baicalin is placed. The carbon dioxide is pressurized and heated to its supercritical state and then passed through the extraction vessel. The separator is used to separate the extracted baicalin from the supercritical carbon dioxide, and the collection unit is used to collect the final product.6.2. Pretreatment of Plant Material
Before the extraction, the plant material needs to be properly pretreated. This may include drying, grinding, and sieving. Drying the plant material can reduce its moisture content, which can affect the extraction efficiency. Grinding the plant material can increase its surface area, facilitating the contact between the plant material and supercritical carbon dioxide. Sieving can ensure that the particle size of the plant material is within a suitable range for extraction.6.3. Optimization of Extraction Conditions
As mentioned before, the extraction conditions such as pressure, temperature, extraction time, and the use of co - solvents need to be optimized. This can be achieved through experimental design methods such as response surface methodology. By conducting a series of experiments with different combinations of extraction conditions, an optimal set of conditions can be determined to maximize the extraction yield and quality of baicalin.7. Future Prospects
7.1. Industrial Scale - up
Although SC - CO₂ extraction has shown great potential for the extraction of baicalin at the laboratory scale, its industrial - scale application still faces some challenges. One of the main challenges is the high cost of equipment and operation. However, with the development of technology and the increasing demand for high - quality natural products, it is expected that the cost will gradually decrease in the future. Another aspect to be considered is the scale - up of the extraction process. Optimization of the extraction process on a large scale needs to be further studied to ensure the consistent quality and high yield of baicalin extraction.7.2. Combination with Other Technologies
The combination of SC - CO₂ extraction with other technologies may open up new possibilities for the extraction and purification of baicalin. For example, coupling SC - CO₂ extraction with membrane separation technology can further improve the purity of the extracted baicalin. Also, the combination with supercritical fluid chromatography can be used for the analysis and purification of baicalin at the same time.7.3. New Applications
With the in - depth study of baicalin, new applications may be discovered in the future. SC - CO₂ extraction, with its ability to produce high - quality baicalin, can play an important role in these new applications. For example, baicalin may be used in the development of new drugs for the treatment of chronic diseases, and SC - CO₂ extraction can ensure the purity and bioactivity of baicalin required for pharmaceutical applications.8. Conclusion
Supercritical carbon dioxide extraction of baicalin is a promising technique with many advantages over traditional extraction methods. It can provide high - quality baicalin with high extraction yield, while being environmentally friendly. By understanding the factors affecting the extraction, the mechanisms involved, and the operational aspects, further optimization of the extraction process can be achieved. Looking ahead, the industrial - scale application, combination with other technologies, and new applications of SC - CO₂ extraction of baicalin hold great potential for the development of the pharmaceutical and nutraceutical industries.
FAQ:
What are the advantages of using supercritical carbon dioxide extraction for baicalin?
Supercritical carbon dioxide extraction for baicalin has several advantages. Firstly, due to the high diffusivity and low viscosity of supercritical carbon dioxide, it enables efficient extraction, which can improve the extraction yield and quality. Secondly, compared to conventional solvent extraction, it reduces solvent residue, which is very important for pharmaceutical products. Moreover, it can extract baicalin in a more targeted way, effectively separating it from other components in the plant matrix.
How can the supercritical carbon dioxide extraction process of baicalin be optimized?
The supercritical carbon dioxide extraction process of baicalin can be optimized by controlling factors such as pressure, temperature, and extraction time. Adjusting these parameters appropriately can enhance the extraction efficiency and the quality of the extracted baicalin.
What is the significance of baicalin in medicinal applications?
Baicalin is an important bioactive component. It has great potential in various medicinal applications, which makes the extraction of high - quality baicalin very important. And supercritical carbon dioxide extraction is a promising method to obtain baicalin for medicinal use.
Why is supercritical carbon dioxide extraction more effective in separating baicalin from other plant components?
Supercritical carbon dioxide extraction is more effective in separating baicalin from other plant components because it can be adjusted to target specific components. The properties of supercritical carbon dioxide, such as its diffusivity and solubility characteristics, can be exploited to selectively extract baicalin while leaving other unwanted components behind.
How does supercritical carbon dioxide extraction compare to other extraction methods for baicalin in terms of yield?
Supercritical carbon dioxide extraction can often achieve a relatively high yield of baicalin. Compared to some traditional extraction methods, its high diffusivity and the ability to optimize extraction conditions (such as pressure, temperature, and time) contribute to a better extraction efficiency, resulting in a potentially higher yield of baicalin.
Related literature
- Supercritical Fluid Extraction of Baicalin: A Review of Process Optimization"
- "Advances in Supercritical Carbon Dioxide Extraction of Bioactive Compounds from Medicinal Plants: Focus on Baicalin"
- "The Role of Supercritical CO₂ in the Selective Extraction of Baicalin and Its Purity Enhancement"
- ▶ Hesperidin
- ▶ Citrus Bioflavonoids
- ▶ Plant Extract
- ▶ lycopene
- ▶ Diosmin
- ▶ Grape seed extract
- ▶ Sea buckthorn Juice Powder
- ▶ Fruit Juice Powder
- ▶ Hops Extract
- ▶ Artichoke Extract
- ▶ Mushroom extract
- ▶ Astaxanthin
- ▶ Green Tea Extract
- ▶ Curcumin
- ▶ Horse Chestnut Extract
- ▶ Other Product
- ▶ Boswellia Serrata Extract
- ▶ Resveratrol
- ▶ Marigold Extract
- ▶ Grape Leaf Extract
- ▶ New Product
- ▶ Aminolevulinic acid
- ▶ Cranberry Extract
- ▶ Red Yeast Rice
- ▶ Red Wine Extract
-
Black Garlic Extract
2024-11-30
-
Quercetin
2024-11-30
-
Angelica sinensis extract
2024-11-30
-
Artichoke Leaf Extract
2024-11-30
-
Wheat Germ Extract
2024-11-30
-
Diosmin
2024-11-30
-
Andrographis Paniculata Extract Powder
2024-11-30
-
Plantain extract
2024-11-30
-
Saffron Extract Powder
2024-11-30
-
Genistein
2024-11-30