- 0086-571-85302990
- sales@greenskybio.com
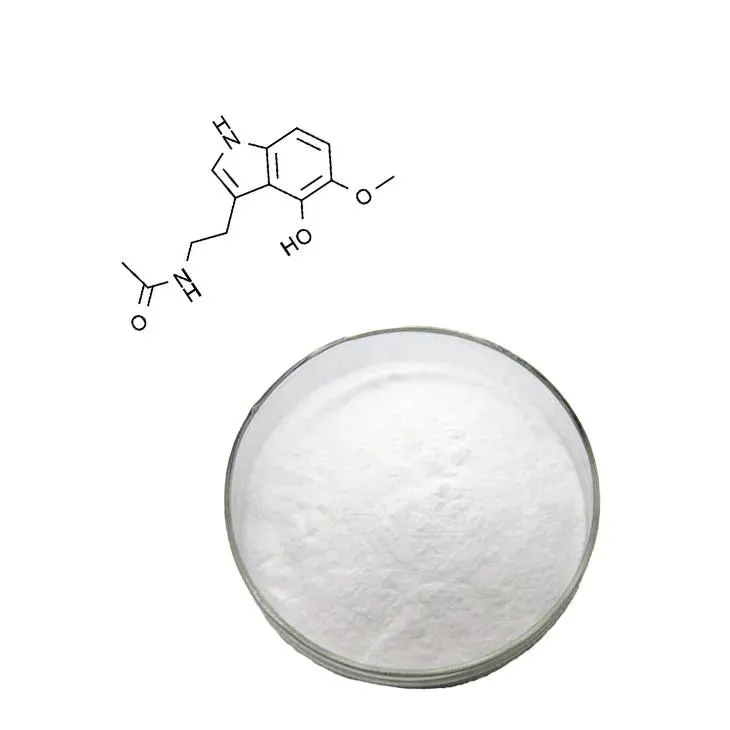
Supercritical Carbon Dioxide Extraction of N - Acetyl - L - Cysteine (NAC).
2024-11-28
1. Introduction
N - acetyl - L - cysteine (NAC) is a significant amino acid derivative that has been drawing increasing attention in various fields due to its multiple biological activities. It plays crucial roles in areas such as antioxidant defense, mucolytic action, and liver protection. Traditionally, extraction methods for NAC have certain limitations, which may lead to issues like low extraction efficiency, product degradation, and high energy consumption. However, supercritical carbon dioxide extraction has emerged as a cutting - edge technology in this regard, with the potential to overcome these drawbacks and transform the way NAC is obtained.
2. The Principle of Supercritical Carbon Dioxide
Carbon dioxide (CO₂) under specific temperature and pressure conditions can reach a supercritical state. In this state, CO₂ exhibits unique properties that make it an excellent solvent for extraction. Supercritical CO₂ has a density similar to that of a liquid, which allows it to dissolve a wide range of substances effectively. At the same time, it has a viscosity and diffusivity closer to that of a gas, enabling it to penetrate into complex matrices quickly and easily.
2.1. Phase Diagram
The phase diagram of CO₂ is fundamental to understanding its supercritical behavior. It shows the relationship between temperature, pressure, and the phase of CO₂ (solid, liquid, or gas). The critical point of CO₂ is at a temperature of approximately 31.1 °C and a pressure of about 7.38 MPa. Beyond this critical point, CO₂ enters the supercritical state. This knowledge is crucial for precisely controlling the extraction process using supercritical CO₂.
3. Supercritical Carbon Dioxide Extraction of NAC
The extraction of NAC using supercritical CO₂ is a complex yet highly efficient process. Firstly, the raw materials containing NAC are prepared. These raw materials can be of various sources, such as natural products or synthetic precursors.
3.1. Extraction Parameters
- Temperature: The choice of temperature is critical. It affects the solubility of NAC in supercritical CO₂. Generally, a specific temperature range is determined based on the nature of the raw materials and the desired extraction efficiency. For example, a temperature slightly above the critical temperature of CO₂ may be selected to enhance the solubility of NAC while avoiding thermal degradation.
- Pressure: Pressure also plays a vital role. Higher pressures usually increase the density of supercritical CO₂, thereby enhancing its solvent power. However, excessive pressure may lead to equipment problems and higher costs. Therefore, an optimal pressure value needs to be determined through experiments, typically ranging from a few MPa to several tens of MPa.
- Flow Rate of CO₂: The flow rate of supercritical CO₂ affects the mass transfer rate during the extraction process. A proper flow rate ensures sufficient contact between CO₂ and the raw materials, facilitating the dissolution and extraction of NAC. If the flow rate is too slow, the extraction time may be prolonged; if it is too fast, the efficiency may not be fully utilized.
3.2. Extraction Process
Once the extraction parameters are set, the supercritical CO₂ is pumped into the extraction vessel containing the raw materials. The supercritical CO₂ penetrates into the matrix of the raw materials, selectively dissolving NAC. Then, the CO₂ - NAC mixture is transferred to a separation vessel, where the pressure and/or temperature are adjusted to cause the CO₂ to return to a gaseous state, leaving behind the extracted NAC. This process can be repeated multiple times to improve the extraction yield.
4. Advantages of Supercritical Carbon Dioxide Extraction for NAC
- Energy - Efficiency: Compared to traditional extraction methods such as solvent extraction or distillation, supercritical CO₂ extraction is relatively energy - efficient. Since the phase transition of CO₂ can be precisely controlled, less energy is wasted during the extraction process.
- Sustainable Production: CO₂ is a non - toxic, non - flammable, and readily available gas. Using supercritical CO₂ for extraction is more environmentally friendly, contributing to sustainable production of NAC. Moreover, the CO₂ can be recycled and reused in the extraction process, further reducing environmental impact.
- High - Quality Product: The purity and bioactivity of the extracted NAC can be well - maintained. Supercritical CO₂ extraction is a relatively mild process, which reduces the risk of chemical reactions or degradations that may occur during extraction. This is highly desirable for applications in pharmaceuticals and nutraceuticals, where high - quality and bioactive ingredients are crucial.
- Selectivity: Supercritical CO₂ can be adjusted to have different solvent properties by changing the extraction parameters. This enables it to selectively extract NAC from complex matrices, separating it from other components effectively. This selectivity can simplify the purification process of NAC and reduce the production cost.
5. Challenges and Solutions in Supercritical Carbon Dioxide Extraction of NAC
- Equipment Cost: The equipment required for supercritical CO₂ extraction, such as high - pressure pumps, extraction vessels, and separation vessels, is relatively expensive. This high initial investment may be a barrier for some small - scale producers. To address this issue, efforts can be made to develop more cost - effective equipment or to share extraction facilities among multiple producers.
- Low Solubility in Some Cases: Although supercritical CO₂ has good solvent properties, in some cases, the solubility of NAC may still be relatively low. This can be improved by adding co - solvents. Co - solvents can enhance the solubility of NAC in supercritical CO₂ without significantly affecting the advantages of the extraction method. However, the selection and use of co - solvents need to be carefully studied to ensure compliance with safety and quality requirements.
- Process Optimization: The extraction process needs to be continuously optimized to achieve higher extraction yields and better product quality. This requires in - depth research on the interaction between supercritical CO₂, NAC, and raw materials, as well as on the influence of various extraction parameters. Through experimental design and data analysis, the optimal extraction conditions can be determined more accurately.
6. Applications of NAC Extracted by Supercritical Carbon Dioxide
- Pharmaceutical Industry: In the pharmaceutical field, NAC has been used for the treatment of various diseases. For example, it is used as a mucolytic agent in respiratory diseases, helping to break down mucus and relieve symptoms. The high - quality NAC extracted by supercritical CO₂ can be formulated into more effective drugs, ensuring its purity and bioactivity.
- Nutraceuticals: NAC is also a popular ingredient in nutraceuticals due to its antioxidant properties. It can be added to dietary supplements to help protect cells from oxidative damage. The pure and bioactive NAC obtained through supercritical CO₂ extraction is ideal for use in nutraceutical products, providing consumers with high - quality health - promoting products.
- Cosmetics: In the cosmetics industry, NAC can be used for its antioxidant and skin - conditioning effects. It can be incorporated into creams, lotions, and serums to help improve skin health and appearance. Supercritical CO₂ - extracted NAC can offer better quality and performance in cosmetics applications.
7. Conclusion
Supercritical carbon dioxide extraction of N - acetyl - L - cysteine (NAC) is a promising technology with numerous advantages. It offers an energy - efficient, sustainable, and high - quality approach to obtaining NAC, which has wide - ranging applications in pharmaceuticals, nutraceuticals, and cosmetics. Although there are some challenges in the process, such as equipment cost and solubility issues, continuous research and development are expected to overcome these problems. As the demand for high - quality NAC continues to grow, supercritical CO₂ extraction is likely to play an increasingly important role in the future production of NAC.
FAQ:
What are the advantages of supercritical carbon dioxide extraction for N - acetyl - L - cysteine (NAC)?
The advantages are numerous. Firstly, it can efficiently separate NAC from complex matrices. Secondly, it is an energy - efficient method, which is beneficial for sustainable production. Moreover, the purity and bioactivity of the extracted NAC can be well - maintained, making it highly suitable for applications in pharmaceuticals and nutraceuticals.
What are the specific temperature and pressure conditions for supercritical CO₂ extraction of NAC?
The specific temperature and pressure conditions are those where carbon dioxide exhibits supercritical behavior. However, the exact values can vary depending on the nature of the sample and the extraction system used. Generally, the temperature is typically above the critical temperature of CO₂ (31.1 °C) and the pressure is above its critical pressure (73.8 bar), but more precise values need to be determined through experimental optimization for NAC extraction.
Why is maintaining the purity and bioactivity of NAC important?
Maintaining the purity and bioactivity of NAC is crucial because in pharmaceutical applications, high - purity NAC is required to ensure its efficacy and safety. In nutraceuticals, the bioactivity of NAC is directly related to its health - promoting properties. If the purity is low or the bioactivity is lost during extraction, it may not be able to perform its intended functions effectively, such as antioxidant, mucolytic, or anti - inflammatory functions.
How does supercritical CO₂ extraction contribute to sustainable production of NAC?
Supercritical CO₂ extraction contributes to sustainable production in several ways. Since it is energy - efficient, it reduces the overall energy consumption compared to some traditional extraction methods. Also, carbon dioxide is a non - toxic, non - flammable, and easily available solvent. After the extraction process, the CO₂ can be easily recovered and recycled, reducing waste and environmental impact.
What are the challenges in supercritical CO₂ extraction of NAC?
One of the challenges can be the high initial investment required for the extraction equipment that can operate under supercritical conditions. Another challenge is the optimization of extraction parameters, such as temperature, pressure, and extraction time, which may need extensive experimentation. Also, compared to some other solvents, the solubility of NAC in supercritical CO₂ may be relatively low in some cases, which may require additional techniques or modifiers to enhance the extraction efficiency.
Related literature
- Supercritical Fluid Extraction of Bioactive Compounds"
- "Advances in Supercritical Carbon Dioxide Extraction Technology for Pharmaceuticals"
- "N - Acetyl - L - Cysteine: Properties, Synthesis, and Applications"
TAGS:
Why is maintaining the purity and bioactivity of NAC important?
Maintaining the purity and bioactivity of NAC is crucial because in pharmaceutical applications, high - purity NAC is required to ensure its efficacy and safety. In nutraceuticals, the bioactivity of NAC is directly related to its health - promoting properties. If the purity is low or the bioactivity is lost during extraction, it may not be able to perform its intended functions effectively, such as antioxidant, mucolytic, or anti - inflammatory functions.
How does supercritical CO₂ extraction contribute to sustainable production of NAC?
Supercritical CO₂ extraction contributes to sustainable production in several ways. Since it is energy - efficient, it reduces the overall energy consumption compared to some traditional extraction methods. Also, carbon dioxide is a non - toxic, non - flammable, and easily available solvent. After the extraction process, the CO₂ can be easily recovered and recycled, reducing waste and environmental impact.
What are the challenges in supercritical CO₂ extraction of NAC?
One of the challenges can be the high initial investment required for the extraction equipment that can operate under supercritical conditions. Another challenge is the optimization of extraction parameters, such as temperature, pressure, and extraction time, which may need extensive experimentation. Also, compared to some other solvents, the solubility of NAC in supercritical CO₂ may be relatively low in some cases, which may require additional techniques or modifiers to enhance the extraction efficiency.
Related literature
- Supercritical Fluid Extraction of Bioactive Compounds"
- "Advances in Supercritical Carbon Dioxide Extraction Technology for Pharmaceuticals"
- "N - Acetyl - L - Cysteine: Properties, Synthesis, and Applications"
TAGS:
What are the challenges in supercritical CO₂ extraction of NAC?
One of the challenges can be the high initial investment required for the extraction equipment that can operate under supercritical conditions. Another challenge is the optimization of extraction parameters, such as temperature, pressure, and extraction time, which may need extensive experimentation. Also, compared to some other solvents, the solubility of NAC in supercritical CO₂ may be relatively low in some cases, which may require additional techniques or modifiers to enhance the extraction efficiency.
Related literature
- Supercritical Fluid Extraction of Bioactive Compounds"
- "Advances in Supercritical Carbon Dioxide Extraction Technology for Pharmaceuticals"
- "N - Acetyl - L - Cysteine: Properties, Synthesis, and Applications"
TAGS:
- ▶ Hesperidin
- ▶ citrus bioflavonoids
- ▶ plant extract
- ▶ lycopene
- ▶ Diosmin
- ▶ Grape seed extract
- ▶ Sea buckthorn Juice Powder
- ▶ Beetroot powder
- ▶ Hops Extract
- ▶ Artichoke Extract
- ▶ Reishi mushroom extract
- ▶ Astaxanthin
- ▶ Green Tea Extract
- ▶ Curcumin Extract
- ▶ Horse Chestnut Extract
- ▶ Other Problems
- ▶ Boswellia Serrata Extract
- ▶ Resveratrol Extract
- ▶ Marigold Extract
- ▶ Grape Leaf Extract
- ▶ blog3
- ▶ blog4
- ▶ blog5
-
The best lemon juice powder in nature.
2024-11-28
-
Organic Vitamin K2 Powder Suppliers
2024-11-28
-
Bulk purchase of L - tyrosine.
2024-11-28
-
Vitamin K2 Manufacturers
2024-11-28
-
100% Pure Natural Rutin.
2024-11-28
-
Chinese Citrus Bioflavonoid Suppliers.
2024-11-28
-
Artichoke Extract
2024-11-28
-
Citrus bioflavonoids
2024-11-28
-
Tongkat Ali Extract Powder
2024-11-28
-
melatonin extract
2024-11-28
-
Okra Extract
2024-11-28
-
Genistein
2024-11-28
-
Selenium yeast
2024-11-28
-
Horse Chestnut Extract
2024-11-28
-
Nettle leaf extract
2024-11-28
-
Angelica sinensis extract
2024-11-28