- 0086-571-85302990
- sales@greenskybio.com
Preparation process of quercetin.
2024-12-15

1. Introduction to Quercetin
Quercetin is a remarkable natural flavonoid that has been the focus of extensive research in recent years. It is widely distributed in nature, especially in various plants. Quercetin is known for its diverse biological activities, which include antioxidant, anti - inflammatory, anti - cancer, and cardiovascular protection properties. Due to these beneficial effects, there is a growing demand for the preparation of high - quality quercetin for applications in medicine, food, and cosmetics industries.

2. Selection of Raw Materials
The first step in the preparation of quercetin is the careful selection of raw materials. Since quercetin is a natural compound, plants are the primary source of its precursors.
2.1 Common Plant Sources
- Onions: Onions are a well - known source of quercetin. They contain a significant amount of flavonoids, with quercetin being one of the major components. The outer layers of onions are particularly rich in quercetin. - Apples: Apples also contain quercetin, especially in the peel. The peel of apples contains a relatively high concentration of flavonoids, and quercetin contributes to the antioxidant properties associated with apples. - Berries: Many types of berries, such as cranberries, blueberries, and strawberries, are rich sources of quercetin. These berries are not only delicious but also offer a natural source of this valuable flavonoid.
2.2 Considerations for Raw Material Selection
When selecting raw materials for quercetin extraction, several factors need to be considered:
- Quercetin Content: The amount of quercetin present in the plant material is crucial. Higher - quercetin - containing plants are preferred as they can yield more of the desired compound during extraction.
- Availability and Cost: The availability of the plant material on a large scale and its cost are important economic factors. Raw materials that are widely available and cost - effective are more suitable for large - scale quercetin production.
- Purity and Contaminants: The purity of the raw material is essential. Plants should be free from excessive pesticides, heavy metals, and other contaminants that could affect the quality of the extracted quercetin.

3. Extraction Processes
Once the appropriate raw materials are selected, the next step is extraction. The goal of extraction is to obtain a solution containing the flavonoid - rich components, including quercetin, from the plant material.
3.1 Solvent Extraction
Solvent extraction is one of the most commonly used methods for quercetin extraction. Ethanol and methanol are two frequently employed solvents for this purpose. - Principle: The principle behind solvent extraction is based on the solubility of quercetin in the chosen solvent. Quercetin has a certain solubility in polar solvents like ethanol and methanol. When the plant material is immersed in the solvent, the quercetin molecules dissolve into the solvent, forming a solution. - Procedure: 1. First, the plant material is ground into a fine powder. This increases the surface area of the material, facilitating better contact with the solvent. 2. Then, a suitable amount of the solvent (ethanol or methanol) is added to the powdered plant material. The ratio of solvent to plant material is an important parameter and needs to be optimized depending on the type of plant and the expected quercetin yield. 3. The mixture is then stirred or shaken for a specific period, usually several hours to ensure complete extraction. This can be done at room temperature or under mild heating conditions to enhance the extraction efficiency. 4. After extraction, the mixture is filtered to separate the liquid extract (containing quercetin) from the solid residue of the plant material.
3.2 Other Extraction Methods
- Supercritical Fluid Extraction: Supercritical fluid extraction (SFE) is an emerging extraction technique. Carbon dioxide (CO₂) is often used as the supercritical fluid. The advantage of SFE is that it can operate at relatively low temperatures, which helps to preserve the thermally labile components of the plant material. However, the equipment for SFE is more expensive compared to solvent extraction methods. - Microwave - Assisted Extraction: This method utilizes microwave energy to enhance the extraction process. Microwave - assisted extraction can significantly reduce the extraction time. The microwaves heat the plant - solvent mixture rapidly and uniformly, increasing the mass transfer rate of quercetin from the plant material to the solvent.
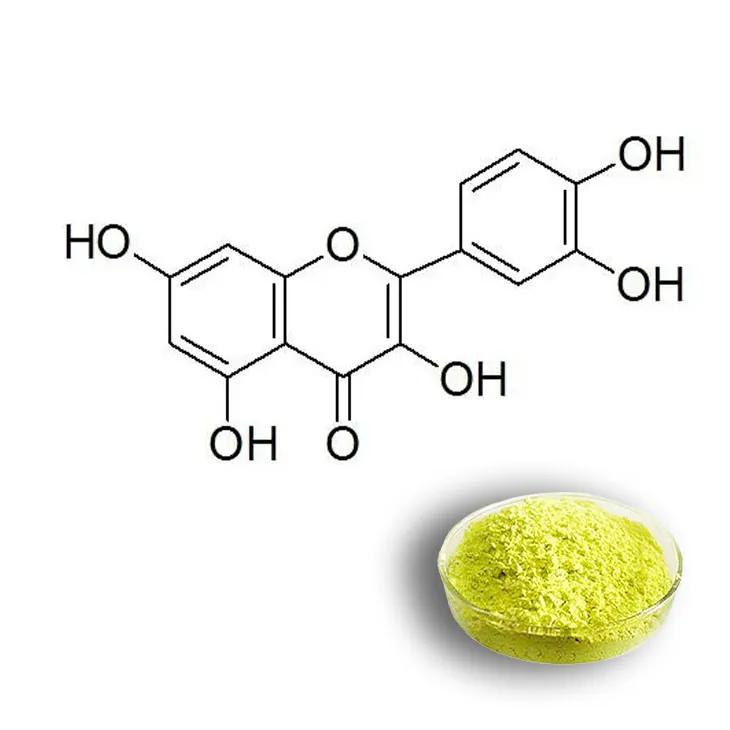
4. Purification Steps
After extraction, the obtained extract contains not only quercetin but also other substances such as other flavonoids, sugars, and pigments. Therefore, purification steps are necessary to obtain pure quercetin.
4.1 Chromatography
Chromatography is a powerful technique for purifying quercetin. Among the various chromatography methods, high - performance liquid chromatography (HPLC) is widely used. - Principle of HPLC: HPLC separates components based on their differential interactions with a stationary phase and a mobile phase. In the case of quercetin purification, the sample (the extract containing quercetin) is injected into the HPLC system. The mobile phase, which is a liquid solvent (such as a mixture of water and acetonitrile), carries the sample through a column filled with a stationary phase (usually a silica - based material). Quercetin and other components in the sample interact differently with the stationary and mobile phases, resulting in different retention times. This allows the separation of quercetin from other substances. - Operation of HPLC: 1. The HPLC system is first calibrated using standard quercetin solutions to establish accurate retention times and peak areas for quantification. 2. The sample extract is then injected into the HPLC system. The injection volume is carefully controlled to ensure accurate analysis. 3. As the sample travels through the column, the detector (such as a UV - Vis detector) monitors the eluted components. The detector records the signal corresponding to the presence of quercetin, and based on the retention time and peak area, the purity and quantity of quercetin in the sample can be determined.
4.2 Crystallization
Crystallization is another common method for obtaining pure quercetin. - Principle: Crystallization is based on the solubility difference of quercetin in a solvent at different temperatures. By adjusting the temperature and concentration of the quercetin - containing solution, quercetin can be made to crystallize out of the solution. - Procedure: 1. The quercetin - rich extract obtained after extraction and partial purification (if any) is concentrated to a suitable concentration. This can be done by evaporating the solvent under reduced pressure or gentle heating. 2. Then, the concentrated solution is cooled slowly. As the temperature decreases, the solubility of quercetin in the solvent decreases, and quercetin crystals start to form. 3. The formed crystals are then separated from the mother liquor by filtration or centrifugation. The obtained crystals are relatively pure quercetin.

5. Quality Control and Characterization
After the preparation of quercetin, quality control and characterization are essential to ensure that the final product meets the required standards for its intended applications.
5.1 Quality Control
- Purity Analysis: The purity of quercetin is determined using methods such as HPLC. A high - purity quercetin product is required for applications in medicine and cosmetics. The purity should typically be above a certain percentage (e.g., 95% or higher depending on the application). - Contaminant Detection: Detection of contaminants such as heavy metals (e.g., lead, mercury), pesticides, and residual solvents is crucial. These contaminants can pose risks to human health if present in the quercetin product. Analytical techniques like atomic absorption spectroscopy for heavy metals and gas chromatography for residual solvents are used for contaminant detection.
5.2 Characterization
- Spectroscopic Analysis: Spectroscopic techniques such as ultraviolet - visible (UV - Vis) spectroscopy, infrared (IR) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy are used to characterize quercetin. UV - Vis spectroscopy can provide information about the absorption properties of quercetin, which is related to its structure and antioxidant activity. IR spectroscopy can be used to identify the functional groups present in quercetin, and NMR spectroscopy can provide detailed structural information about the molecule. - Melting Point Determination: The melting point of quercetin is a characteristic physical property. Determining the melting point can help in verifying the identity and purity of the quercetin sample. A pure quercetin sample should have a specific melting point within a narrow range.
6. Conclusion
The preparation of quercetin involves a series of well - designed steps from the selection of raw materials to extraction, purification, and quality control. Each step is crucial in obtaining high - quality quercetin for its diverse applications in medicine, food, and cosmetics. With the increasing demand for natural bioactive compounds, continuous research and improvement in the preparation process of quercetin are expected to ensure its efficient production and reliable quality.
FAQ:
What are the common raw materials for quercetin preparation?
Common raw materials for quercetin preparation are certain plants that are rich in quercetin precursors.
Why are ethanol and methanol often used in the extraction process?
Ethanol and methanol are often used as solvents in the extraction process because they are effective in extracting the flavonoid - rich components from the plant materials.
What is the role of chromatography in quercetin preparation?
Chromatography, such as high - performance liquid chromatography (HPLC), plays a crucial role in quercetin preparation. It is used to separate and purify quercetin from other substances.
How does crystallization help in obtaining pure quercetin?
Crystallization is a common method to obtain pure quercetin crystals. During crystallization, quercetin molecules come together in an ordered manner to form pure crystals, separating from impurities.
What are the applications of quercetin in different fields?
Quercetin has various applications in the fields of medicine, food, and cosmetics. In medicine, it may have potential health - promoting effects. In food, it can be used as a natural antioxidant. In cosmetics, it may contribute to skin - related benefits.
Related literature
- Preparation and Characterization of Quercetin - Loaded Nanoparticles for Biomedical Applications"
- "Optimization of Quercetin Extraction from Plant Sources"
- "Quercetin: A Review of Its Bioactivity and Potential Applications in Food and Pharmaceutical Industries"
- ▶ Hesperidin
- ▶ citrus bioflavonoids
- ▶ plant extract
- ▶ lycopene
- ▶ Diosmin
- ▶ Grape seed extract
- ▶ Sea buckthorn Juice Powder
- ▶ Beetroot powder
- ▶ Hops Extract
- ▶ Artichoke Extract
- ▶ Reishi mushroom extract
- ▶ Astaxanthin
- ▶ Green Tea Extract
- ▶ Curcumin Extract
- ▶ Horse Chestnut Extract
- ▶ Other Problems
- ▶ Boswellia Serrata Extract
- ▶ Resveratrol Extract
- ▶ Marigold Extract
- ▶ Grape Leaf Extract
- ▶ blog3
- ▶ blog4
- ▶ blog5
-
Seven Trends of Nettle Leaf Extracts.
2024-12-15
-
Artichoke Extract
2024-12-15
-
Chaste Berry Extract
2024-12-15
-
Oyster Mushroom Extract Powder
2024-12-15
-
Acerola Extract
2024-12-15
-
Epimedium extract powder
2024-12-15
-
Elderberry Extract
2024-12-15
-
Fenugreek Extract Powder
2024-12-15
-
Kidney Bean Extract
2024-12-15
-
Alisma Extract
2024-12-15
-
Echinacea Extract
2024-12-15





















