- 0086-571-85302990
- sales@greenskybio.com
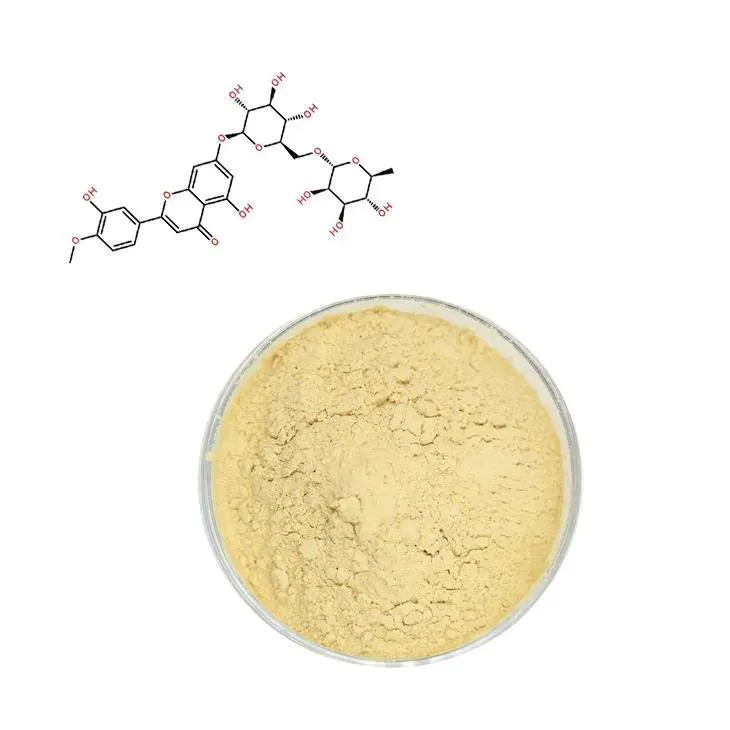
Supercritical carbon dioxide extraction of diosmin.
2024-12-01

1. Introduction to Diosmin
Diosmin is a flavonoid compound that has been widely recognized for its significant biological activities. It is particularly well - known for its anti - inflammatory, antioxidant and vein - protecting effects. In the medical field, these properties make it a valuable compound for the treatment of various conditions related to venous insufficiency, such as varicose veins, hemorrhoids, and chronic venous leg ulcers. Diosmin can also play a role in reducing inflammation in other parts of the body, which may be beneficial in the management of certain inflammatory diseases. Moreover, its antioxidant activity helps to protect cells from oxidative damage caused by free radicals, which is implicated in many age - related diseases and degenerative processes.
2. Traditional extraction methods of Diosmin
Before the advent of supercritical carbon dioxide extraction, traditional extraction methods were mainly used to obtain Diosmin from plant sources. One of the most common traditional methods is solvent extraction.
2.1 Solvent extraction
Solvent extraction typically involves the use of organic solvents such as ethanol, methanol or acetone. The plant material containing Diosmin is soaked in the solvent for a certain period of time, allowing the Diosmin to dissolve into the solvent. Then, through filtration and evaporation processes, the solvent is removed to obtain the Diosmin extract.
However, this method has several drawbacks. Firstly, there is a high probability of solvent residue remaining in the final product. For pharmaceutical applications, even a small amount of solvent residue can be unacceptable as it may have potential toxic effects on the human body. Secondly, the extraction process using solvents may not be very selective, which means that other unwanted compounds may also be extracted along with Diosmin, leading to a lower purity of the final product.
3. Supercritical carbon dioxide extraction technology
Supercritical carbon dioxide extraction is a relatively new and advanced extraction technology that has shown great potential in the extraction of Diosmin.
3.1 Properties of supercritical carbon dioxide
Carbon dioxide (CO₂) under supercritical conditions exhibits unique properties. Supercritical CO₂ has a density similar to that of a liquid, which allows it to have good solvating power. At the same time, it has the diffusivity and low viscosity characteristics of a gas, enabling it to penetrate easily into the raw materials. These properties make supercritical CO₂ an ideal solvent for the extraction of Diosmin.
3.2 The extraction process
The supercritical CO₂ extraction process for Diosmin typically involves the following steps:
- First, the plant material containing Diosmin is placed in the extraction vessel. The extraction vessel is then pressurized and heated to bring the carbon dioxide to its supercritical state.
- Once in the supercritical state, the CO₂ can selectively dissolve Diosmin from the plant material. This selectivity is due to the specific interactions between the supercritical CO₂ and Diosmin molecules, while leaving behind many of the unwanted components in the plant material.
- After the extraction is complete, the pressure and / or temperature of the system is changed. This causes the supercritical CO₂ to return to a gaseous or liquid state, and Diosmin is separated from the CO₂. The CO₂ can then be recycled for further use in the extraction process.
4. Advantages of supercritical carbon dioxide extraction of Diosmin
There are several notable advantages of using supercritical carbon dioxide extraction for Diosmin compared to traditional extraction methods.
- Low solvent residue: As mentioned earlier, one of the major problems with traditional solvent extraction is solvent residue. In supercritical CO₂ extraction, since CO₂ is a gas at normal conditions, it can be completely removed from the final product, leaving virtually no solvent residue. This is especially important for pharmaceutical applications where high purity and safety are required.
- High selectivity: Supercritical CO₂ can selectively dissolve Diosmin, which means that the purity of the extracted Diosmin can be relatively high. This selectivity reduces the need for further purification steps compared to traditional extraction methods, saving both time and cost.
- Better process control: The supercritical CO₂ extraction process can be precisely controlled by adjusting parameters such as pressure, temperature and flow rate. This allows for the optimization of the extraction yield and quality of Diosmin. For example, by carefully controlling the pressure and temperature, the solubility of Diosmin in supercritical CO₂ can be maximized, resulting in a higher extraction yield.
- Environmentally friendly: Carbon dioxide is a non - toxic, non - flammable and naturally occurring gas. Using supercritical CO₂ as an extraction solvent is more environmentally friendly compared to using organic solvents in traditional extraction methods, which may be harmful to the environment if not properly disposed of.
5. Factors affecting the supercritical carbon dioxide extraction of Diosmin
Several factors can influence the efficiency and quality of the supercritical carbon dioxide extraction of Diosmin.
- Pressure: Pressure is a crucial factor in supercritical CO₂ extraction. Generally, as the pressure increases, the density of supercritical CO₂ also increases, which leads to an increase in its solvating power. However, there is an optimal pressure range for the extraction of Diosmin. If the pressure is too high, it may lead to the extraction of unwanted components along with Diosmin, while if the pressure is too low, the extraction yield may be unsatisfactory.
- Temperature: Temperature also plays an important role. An appropriate temperature can enhance the diffusivity of supercritical CO₂ and the solubility of Diosmin. However, like pressure, there is an optimal temperature range. High temperatures may cause thermal degradation of Diosmin or other components in the plant material, while low temperatures may reduce the extraction efficiency.
- Particle size of raw materials: The particle size of the plant material containing Diosmin affects the extraction efficiency. Smaller particle sizes generally result in a larger surface area, which allows for better contact between the supercritical CO₂ and Diosmin in the plant material. However, if the particle size is too small, it may cause problems such as clogging in the extraction system.
- Extraction time: The extraction time is another factor to consider. Longer extraction times may increase the extraction yield, but it may also lead to the extraction of unwanted components over time. Therefore, an optimal extraction time needs to be determined to balance the extraction yield and the purity of Diosmin.
6. Current research and applications of supercritical carbon dioxide extraction of Diosmin
There has been a growing amount of research on supercritical carbon dioxide extraction of Diosmin in recent years.
6.1 Research progress
Researchers are constantly exploring the optimal extraction conditions for Diosmin, including the determination of the best pressure, temperature, extraction time and other parameters. They are also studying the effects of different plant sources and pretreatment methods of raw materials on the extraction efficiency and quality of Diosmin. For example, some studies have found that different varieties of plants containing Diosmin may have different extraction yields under the same supercritical CO₂ extraction conditions.
6.2 Industrial applications
In the pharmaceutical industry, supercritical carbon dioxide extraction of Diosmin has started to be applied on a small scale. The high - quality Diosmin obtained through this method can be used in the production of drugs for the treatment of venous diseases. In addition, in the field of natural product extraction, supercritical CO₂ extraction of Diosmin is also considered as a promising technology for the production of high - value - added Diosmin - containing products, such as dietary supplements.
7. Conclusion
Supercritical carbon dioxide extraction of Diosmin is a promising technology with many advantages over traditional extraction methods. It offers low solvent residue, high selectivity, better process control and is environmentally friendly. However, there are still some factors that need to be carefully considered and optimized to ensure the highest extraction efficiency and quality of Diosmin. With further research and development, it is expected that supercritical CO₂ extraction will play an increasingly important role in the production of Diosmin - based products in the pharmaceutical and natural product industries.
FAQ:
What are the advantages of supercritical carbon dioxide extraction of diosmin compared to traditional solvent extraction?
The supercritical carbon dioxide extraction of diosmin has several advantages over traditional solvent extraction. Firstly, it has less solvent residue, which is very important for pharmaceutical applications. Secondly, it allows for better control over the extraction process, enabling the optimization of the extraction yield and quality of diosmin. Supercritical CO₂ can easily penetrate into the raw materials and selectively dissolve diosmin, and then separate it through pressure and temperature changes.
How does supercritical carbon dioxide selectively extract diosmin?
Supercritical CO₂ can penetrate into the raw materials easily. Due to its properties, it can selectively dissolve diosmin. Then, by adjusting the pressure and temperature, the dissolved diosmin can be separated. This selectivity is based on the different solubility of components in supercritical CO₂ under different conditions.
Why is supercritical carbon dioxide extraction suitable for pharmaceutical applications in relation to diosmin?
Supercritical carbon dioxide extraction is suitable for pharmaceutical applications regarding diosmin because it has less solvent residue. In the pharmaceutical field, the purity and safety of the extracted substance are crucial. Less solvent residue means a lower risk of contamination, which is essential for drugs. Moreover, it can better control the extraction process to ensure the quality of diosmin obtained.
What factors can affect the extraction yield of diosmin by supercritical carbon dioxide extraction?
Several factors can affect the extraction yield of diosmin by supercritical carbon dioxide extraction. The pressure and temperature are important factors as they influence the solubility of diosmin in supercritical CO₂. The particle size of the raw materials can also have an impact. Smaller particle sizes usually provide a larger surface area for supercritical CO₂ to interact with, which may increase the extraction yield. Additionally, the extraction time can play a role. Longer extraction times may lead to higher yields up to a certain point, but may also cause degradation of diosmin if too long.
Can supercritical carbon dioxide extraction completely replace traditional extraction methods for diosmin?
While supercritical carbon dioxide extraction has many advantages for diosmin extraction, it may not completely replace traditional extraction methods at present. Traditional methods may still be used in some cases due to cost - effectiveness or the availability of equipment. However, with the development of technology and the increasing demand for high - quality and pure diosmin in the pharmaceutical industry, supercritical carbon dioxide extraction is likely to gain more importance and wider application in the future.
Related literature
- Supercritical Fluid Extraction of Diosmin: Process Optimization and Characterization"
- "Advances in Supercritical Carbon Dioxide Extraction of Bioactive Compounds: The Case of Diosmin"
- "Supercritical CO₂ Extraction of Diosmin: A Green and Efficient Technique"
- ▶ Hesperidin
- ▶ citrus bioflavonoids
- ▶ plant extract
- ▶ lycopene
- ▶ Diosmin
- ▶ Grape seed extract
- ▶ Sea buckthorn Juice Powder
- ▶ Beetroot powder
- ▶ Hops Extract
- ▶ Artichoke Extract
- ▶ Reishi mushroom extract
- ▶ Astaxanthin
- ▶ Green Tea Extract
- ▶ Curcumin Extract
- ▶ Horse Chestnut Extract
- ▶ Other Problems
- ▶ Boswellia Serrata Extract
- ▶ Resveratrol Extract
- ▶ Marigold Extract
- ▶ Grape Leaf Extract
- ▶ blog3
- ▶ blog4
-
The best lemon juice powder in nature.
2024-12-01
-
Organic Vitamin K2 Powder Suppliers
2024-12-01
-
Bulk purchase of L - tyrosine.
2024-12-01
-
Vitamin K2 Manufacturers
2024-12-01
-
100% Pure Natural Rutin.
2024-12-01
-
Chinese Citrus Bioflavonoid Suppliers.
2024-12-01
-
Black Garlic Extract
2024-12-01
-
Artichoke Leaf Extract
2024-12-01
-
Dan Shen Root Extract/Salvia Root Extract
2024-12-01
-
Moringa powder
2024-12-01
-
Tamarind extract powder
2024-12-01
-
Resveratrol extract
2024-12-01
-
Curcuma Longa Extract
2024-12-01
-
Acai Berry Extract
2024-12-01
-
Tormentil Extract
2024-12-01
-
Fig Extract
2024-12-01





















