- 0086-571-85302990
- sales@greenskybio.com
Two Bases for the Quality of Diosmin: Potency and Purity
2024-12-21

1. Introduction
Diosmin, a compound of significant importance, has its quality intrinsically linked to two key aspects: potency and purity. These two elements play a crucial role in determining the overall value and effectiveness of Diosmin, whether it is utilized in the medical field or other relevant applications. Understanding the significance of potency and purity is essential for assessing, producing, and using Diosmin-based products.
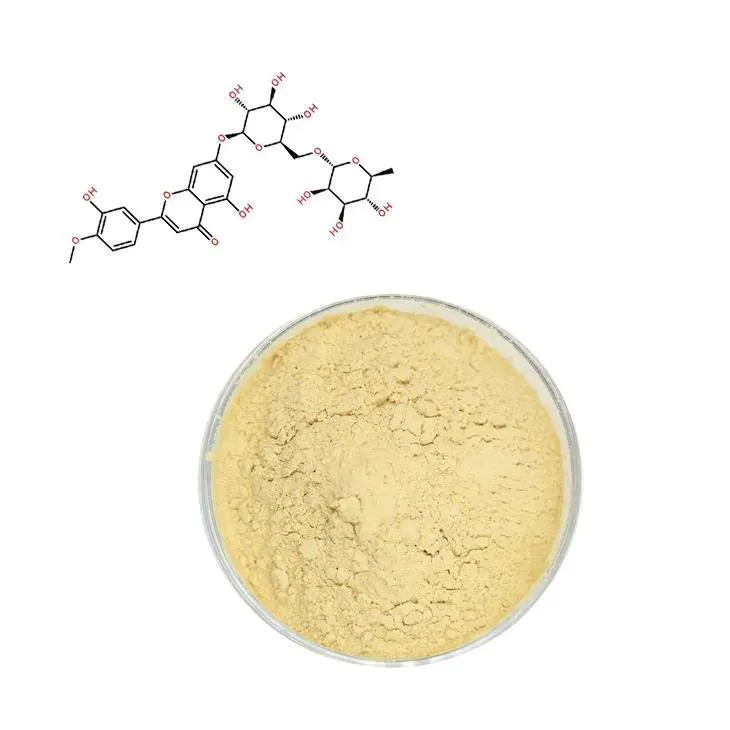
2. Potency of Diosmin
2.1 Definition and Concept
Potency in the context of Diosmin refers to its inherent strength in performing its intended functions. In the medical realm, for example, Diosmin may be used for various therapeutic purposes. A high - potency Diosmin implies that it has a greater ability to bring about the desired physiological or biochemical changes. This could be related to its impact on blood vessels, anti - inflammatory properties, or other functions depending on its application.
2.2 Importance in Medicinal Applications
When used as a medicine, the potency of Diosmin is of utmost importance. For instance, in the treatment of venous disorders, a potent Diosmin can more effectively improve venous tone, reduce capillary permeability, and enhance lymphatic drainage. This is vital for patients suffering from conditions such as varicose veins or chronic venous insufficiency. A more potent form of Diosmin can lead to quicker and more pronounced improvements in symptoms, potentially reducing the duration of treatment and enhancing the patient's quality of life.
Moreover, in the field of anti - inflammatory applications, a high - potency Diosmin can better modulate the body's inflammatory response. It can target specific inflammatory pathways and mediators more efficiently, thereby providing more effective relief from inflammatory - related symptoms. This is especially relevant in conditions where chronic inflammation is a major factor, such as in certain autoimmune disorders or post - surgical inflammation.
2.3 Factors Affecting Potency
Several factors can influence the potency of Diosmin. Source and extraction methods play a significant role. If Diosmin is derived from natural sources, the quality of the source material, such as the species of plant and the environmental conditions in which it is grown, can impact its potency. For example, plants grown in nutrient - rich soil and optimal climatic conditions may produce Diosmin with higher potency. Additionally, the extraction process, whether it is a chemical extraction or a more natural - based extraction method, needs to be carefully optimized to preserve the potency of Diosmin.
Another factor is the formulation and delivery system of Diosmin. If Diosmin is formulated into a particular dosage form, such as a tablet, capsule, or topical cream, the way it is formulated can affect its bioavailability and, consequently, its potency. For example, a well - designed formulation that enhances the absorption of Diosmin in the body will result in a more potent effect. Similarly, the use of appropriate excipients and delivery technologies can also improve the potency of Diosmin - based products.
3. Purity of Diosmin
3.1 Definition and Concept
Purity of Diosmin refers to the degree to which it is free from contaminants. This includes not only chemical impurities but also other substances that may be present during the production process. A pure Diosmin sample should consist almost entirely of Diosmin itself, with minimal amounts of other compounds or elements.
3.2 Significance in Product Quality
The purity of Diosmin has a profound impact on the quality of Diosmin - based products. Firstly, it is crucial for product stability. Impurities in Diosmin can potentially react with Diosmin or other components in the product, leading to degradation or loss of activity over time. A pure Diosmin sample is more likely to remain stable during storage and use, ensuring that the product retains its effectiveness throughout its shelf - life.
Secondly, purity is essential for efficacy. Contaminants can interfere with the action of Diosmin in the body. For example, if there are impurities that bind to the receptors or enzymes that Diosmin is supposed to interact with, it can reduce the efficacy of Diosmin. A high - purity Diosmin product is more likely to deliver the expected therapeutic or functional effects.
3.3 Ensuring Purity
To ensure the purity of Diosmin, strict quality control measures need to be implemented during the production process. This begins with the raw material sourcing. Suppliers of raw materials should be carefully vetted to ensure that they provide high - quality, pure starting materials. For natural sources of Diosmin, proper identification and authentication of the plant sources are necessary to avoid contamination from other similar - looking plants.
During the manufacturing process, purification techniques such as chromatography, crystallization, and filtration should be employed. Chromatography can separate Diosmin from other closely related compounds, while crystallization can help in obtaining pure Diosmin crystals. Filtration is useful for removing particulate impurities. Regular quality testing at various stages of production, using techniques such as high - performance liquid chromatography (HPLC) and mass spectrometry, is also essential to monitor and ensure the purity of Diosmin.
4. Relationship between Potency and Purity
Potency and purity are not independent aspects but are closely related in determining the quality of Diosmin. A high - purity Diosmin is more likely to maintain its potency. Impurities can potentially interact with Diosmin and either enhance or reduce its potency in an unpredictable way. For example, if there are contaminants that have a similar chemical structure to Diosmin, they may compete with Diosmin for binding sites in the body, thereby reducing its effective potency.
On the other hand, a high - potency Diosmin may also be an indication of high purity. If the extraction and production processes are efficient in producing a potent Diosmin, it is often because they are also effective in removing impurities. However, it is important to note that high potency does not always guarantee high purity, and vice versa. Therefore, both aspects need to be carefully evaluated and controlled during the production and quality assessment of Diosmin - based products.
5. Conclusion
In conclusion, potency and purity are two fundamental bases for the quality of Diosmin. Potency determines the effectiveness of Diosmin in fulfilling its functions, whether in the medical or other relevant fields. Purity, on the other hand, ensures the absence of contaminants, which is crucial for product stability and efficacy. Understanding and controlling these two aspects are essential for the production of high - quality Diosmin - based products. Manufacturers need to implement strict quality control measures to ensure both high potency and high purity, while users should also be aware of the importance of these factors when choosing Diosmin - based products.
FAQ:
What is the importance of potency in Diospyros?
Potency in Diospyros is crucial as it determines its strength in performing functions, whether in medicinal applications or other relevant areas. A high - potency Diospyros is preferred because it can lead to better performance.
How does purity affect Diospyros?
Purity has a significant impact on Diospyros. It ensures the absence of contaminants, which is vital for the product. Moreover, it greatly influences the stability and efficacy of Diospyros.
How can one measure the potency of Diospyros?
Measuring the potency of Diospyros can be a complex process. It may involve various scientific methods depending on its intended use. For medicinal Diospyros, it could be measured through in - vitro and in - vivo assays to determine its effectiveness in treating certain conditions or performing its intended biological functions.
What are the common contaminants that can affect the purity of Diospyros?
Common contaminants that can affect the purity of Diospyros may include other substances from the source plant, environmental pollutants, or residues from the extraction and processing procedures. These contaminants can potentially interfere with the performance and safety of Diospyros.
Why are potency and purity considered the core of Diospyros quality?
Potency and purity are considered the core of Diospyros quality because they are fundamental to its functionality, safety, and effectiveness. Potency determines how well Diospyros can perform its functions, while purity ensures that it is free from harmful contaminants and can maintain its stability and efficacy.
Related literature
- Analysis of Potency in Diospyros - Related Compounds"
- "Purity Standards for Diospyros in Medicinal Applications"
- "The Significance of Potency and Purity in Natural Compounds: A Case Study of Diospyros"
- ▶ Hesperidin
- ▶ citrus bioflavonoids
- ▶ plant extract
- ▶ lycopene
- ▶ Diosmin
- ▶ Grape seed extract
- ▶ Sea buckthorn Juice Powder
- ▶ Beetroot powder
- ▶ Hops Extract
- ▶ Artichoke Extract
- ▶ Reishi mushroom extract
- ▶ Astaxanthin
- ▶ Green Tea Extract
- ▶ Curcumin Extract
- ▶ Horse Chestnut Extract
- ▶ Other Problems
- ▶ Boswellia Serrata Extract
- ▶ Resveratrol Extract
- ▶ Marigold Extract
- ▶ Grape Leaf Extract
- ▶ blog3
- ▶ blog4
- ▶ blog5
-
Pure 85% Tomentil Extract.
2024-12-21
-
Uridine-5'-monophosphate Disodium salt
2024-12-21
-
Artichoke Leaf Extract
2024-12-21
-
Buckthorn bark extract
2024-12-21
-
Garcinia Cambogia Extract
2024-12-21
-
Diosmin
2024-12-21
-
Grape Leaf Extract
2024-12-21
-
Feverfew Extract
2024-12-21
-
Rose Hip Extract
2024-12-21
-
Hawthorn powder
2024-12-21
-
Lily extract
2024-12-21




















